IX.3 Substitution rate for neutral mutations tend to be much higher than substitution rate for selectively significant mutations; nevertheless, in genes exposed to intense positive selection, fixation of selectively significant mutations can prevail
In most genes, the substitution rate for synonymous mutations is greater than the substitution rate for nonsynonymous mutations and, on an average, almost attains the values characteristic for DNA sections not coding functional biological products (Fig. IX.2).
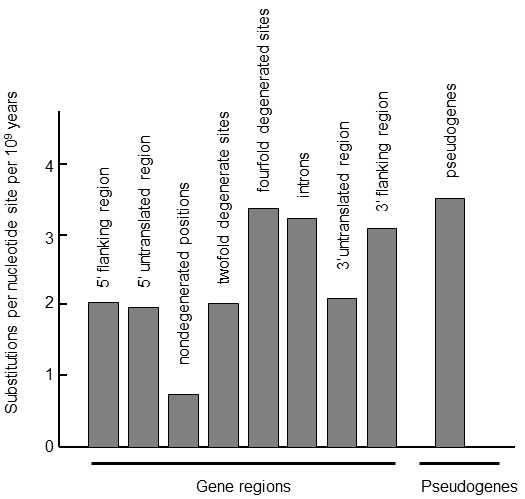
Fig. IX.2. Substitution rate in various regions of genes. Comparison of the genes of humans and mice or rats, or cows and goats, permitted estimation of substitution rates in various regions of genes and pseudogenes. It is apparent from the graph that sequences of pseudogenes change most rapidly in evolution; however, the accumulation of synonymous mutations in the coding sections of genes occurs at almost the same rate, i.e. at positions in which, as a consequence of degeneration of the genetic code, no nucleotide substitution leads to substitution of the amino acid in the final peptide. Intron and 3’-adjacent regions of genes also change relatively rapidly; on the other hand, sections at the 5’-end of the genes, which frequently participate in regulation of gene expression, develop more slowly. Mutations also accumulate more slowly in doubly degenerate positions on the genes, i.e. where substitution of a purine base for some other purine base or a pyrimidine base for some other pyrimidine base does not lead to substitution of an aminoacid, while mutual substitution of a pyrimidine and purine base does lead to substitution of an aminoacid. The sequences in the undegenerated positions on the genes develop most slowly, i.e. where any nucleotide substitution leads to substitution of an aminoacid in the protein chain. According to Li and Graur (1991).
The main reason for reduction of the rate of fixation of nonsynonymous mutations is negative selection (purifying selection), removing from the population mutations that reduce the fitness of their bearers.The vast majority of all mutations fall in this category in a sufficiently large population.Some genes are capable of tolerating a large number of changes without greatly affecting the functioning of the relevant protein.These proteins change very rapidly in evolution.On the other hand, other genes are very conservative and any change in their sequence is greatly manifested in the functioning of the protein and thus in the fitness of the particular individual (Fig. IX.3).
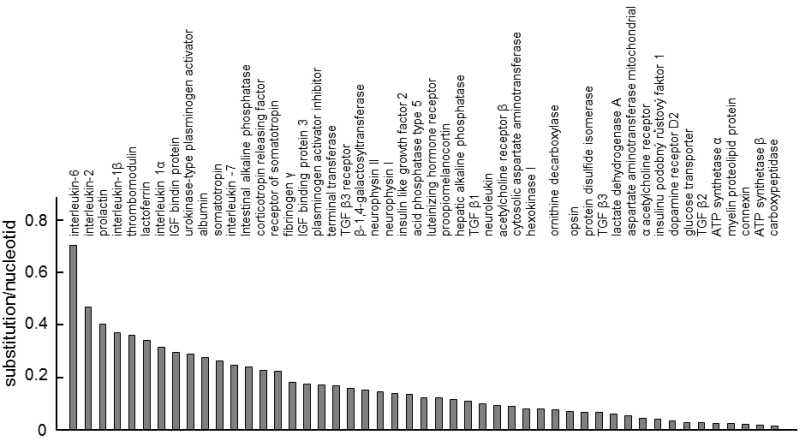
Fig. IX.3. Differences in the rate of evolution between proteins. The graph shows how many synonymous substitutions correspond to the nucleotide for the 49 proteins for which the authors of the study had the sequences from primates, ungulates and rodents at their disposal. It is apparent that the rate of evolution of the individual proteins differs substantially. Data according to Ohta (1995a), modified according to Page and Holmes (2001).
If the intensity and character of selection pressures, to which its representatives are exposed, change during the evolutionary history of a certain taxon, then the intensity of selection acting on the individual genes also changes.If a gene is exposed to more intense selection in a certain period, this is generally manifested by a reduction in the substitution rate for selectively significant mutations.In this case, at a molecular level, a reduction in the overall substitution rate and also a reduction in the ratio of the number of nonsynonymous mutations to the number of synonymous mutations are observed.In both cases, we must relate the numbers of synonymous and nonsynomous mutations to the numbers of positions in which synonymous or nonsynonymous mutations can occur in the given gene.In highly conservative genes exposed to intense negative selection, synonymous mutations substantially predominate and this ratio is thus much lower than 1.From an evolutionary standpoint, the genes for proteins that have a great many functions and that, e.g., interact physically with a large number of other proteins, are very conservative (Fraser et al. 2002).This ratio approaches a value of 1 in DNA sections that usually do not have any function, e.g. in pseudogenes, i.e. in unused and usually incomplete or otherwise damaged copies of genes.In contrast, in genes that are, or were in the past, exposed to intense positive selection, i.e. selection for evolutionary change, nonsynonymous mutations can predominate and this ratio can substantially exceed a value of 1.Genes participating in some way in the co-evolutionary battle amongst parasites and hosts, for example the genes for the components of the immune system, change especially rapidly (Endo, Ikeo, & Gojobori 1996)(Fig. IX.4).Genes that
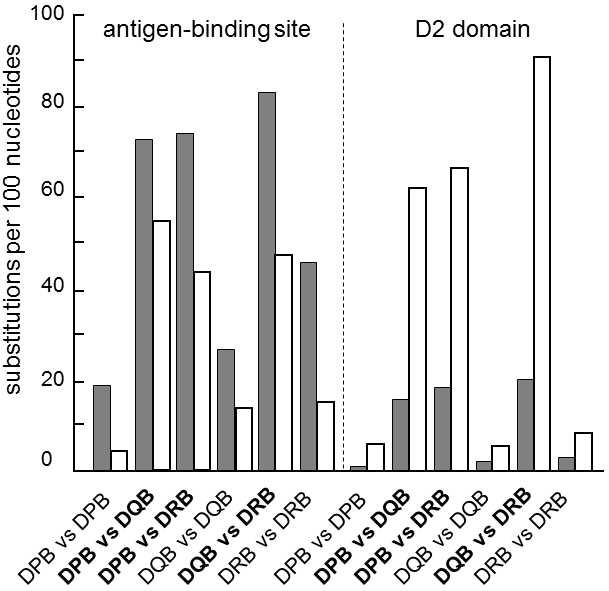
Fig. IX.4. The ratio of the numbers of nonsynonymous and synonymous substitutions in various areas of the MHC II molecule. The graph depicts the numbers of nonsynonymous (dark column) and synonymous (empty column) substitutions per one hundred nonsynonymous and synonymous sites, respectively, in two areas of the MHC II molecule. Both the products of various MHC-genes (bold letters) and various alleles of the same MHC-gene (normal letters) were compared. Nonsynonymous substitutions predominate in the area bonding antigens while, in the other domains, similar to most other proteins, synonymous substitutions predominate. The predominance of nonsynonymous substitutions at antigens binding sites can be most readily explained through the action of positive selection which is probably frequency-dependent selection, because of the relatively high divergence of the individual alleles of a single MHC-gene compared to the divergence of the individual genes (cf. the conditions in the second domain). Data according to Hughes and Nei (1989), modified according to Page and Holmes (2001).
participate in interactions between members of the same species during reproduction, for example receptors on the surface of gametes, proteins expressed in the somatic tissues of the reproductive organs, etc. also develop rapidly (Lee, Ota, & Vacquier 1995; Vacquier 1998; Singh & Kulathinal 2000).This trend is especially important in taxa with polyandrous species in which both intense competition amongst sperms and also stronger genetic conflict between males and females occurs (Panhuis et al. 2001).The ratio of fixed nonsynonymous and synonymous mutations is usually greater than one in these proteins.This situation is encountered in approximately 0.5% of all the proteins that have so far been sequenced.
