XVI. How we unintentionally discovered the function of the Rh factor and probably solved the 50 year-old mystery of Rh polymorphism
Good luck has always played an immensely important role in our studies. I don’t think that it’s true for only our studies – the general significance of luck in science is constantly underestimated. A well-planned study can bring valuable results, but the most crucial results, something entirely novel, are usually stumbled upon when least expected. This is what happened in the case of one of our discoveries – possibly the most important to date – which revealed the influence of the Rh factor on latent toxoplasmosis in humans.
Our good fortune lay in that we studied the effect of toxoplasmosis on the human psyche and performance using several large groups of blood donors. And blood donors, of course, are always tested for their ABO blood group and their Rh factor (Box 68 Blood groups).
Box 68 Blood groupsHumans (as well as other species) have several possible blood groups. This, of course, is crucial information for carrying out blood transfusions and transplants, because a blood transfusion between individuals of different blood groups can easily lead to the receiving patient’s death. The most commonly known blood group system is the ABO system (also written as AB0). People of the blood group A have different sugars (oligosaccharide chains) attached to the outer membrane of each red blood cell (RBC) than do people of the blood group B. People of the AB blood group have both types of sugars on their RBC membranes, and humans of blood group O have neither type. These two types of carbohydrates are also found on the cell walls of certain bacteria, so a human also has antibodies in his blood to act against sugars not present on his own RBCs (of course, a human tolerates his own sugars). When someone with an A or O blood group receives a B or AB blood group transfusion (which I certainly hope is not common nowadays), they experience an acute immune reaction carried out by their antibodies against the foreign blood cells – and most likely die. The same happens when someone with a B or O blood group receives a transfusion of A or AB blood, or when someone with O blood receives a transfusion of any other blood group. A person of the AB blood group is a universal receiver, and can accept any blood; whereas someone of blood group O is a universal donor, and their blood can be accepted by anyone. It’s advantageous for the human species to have several blood groups, with different sugars on their RBC membranes, because this means that people of different blood groups produce different antibodies targeting various structures on the surfaces of parasites – so that they are resistant against different species of parasites. For this reason, parasites cannot easily adapt to the human population, which is useful for our species – always at least part of the population resists the attack of a parasite. Another antigen (a substance which the organism can recognize as foreign and therefore produce antibodies against) that defines human blood groups is the Rh factor. The Rh factor is an antigen capable of inducing the strongest known immune reaction against red blood cells. Unlike antigens of the ABO system, an Rh factor is a protein (actually, just part of protein, called a D antigen), and is not found on bacteria, so unless an Rh negative person comes into contact with foreign Rh positive blood, he won’t have antibodies against the Rh factor. Until recently, neither the influence of the Rh factor on physiological or other human characteristics, nor the biological reason for Rh polymorphism (the presence of Rh positive and negative humans in the population) was known
|
At first we didn’t think that the blood group data from these studies could be useful for something, but since we already had it available, naturally we examined it to see if blood groups correlated either with the presence of toxoplasmosis, or with its behavioral manifestations. Our initial findings weren’t too promising: apparently neither the ABO nor Rh factor blood group played a significant role in the risk of Toxoplasma infection. But later we discovered that the ABO blood group does play a certain role in the probability of Toxoplasma infection. The smallest risk of infection was exhibited by people of the blood group O, and the largest risk by those of blood group AB. But we uncovered this much later, after testing on large subject groups, namely a couple batches of several thousand soldiers each (76).
But let’s get back to our original study conducted on the blood donors tested for toxoplasmosis and blood groups. Like I said, the initial results weren’t to encouraging. The performance of the blood donors did not in any way seem related to their blood group. In the 1999 undergraduate thesis based on this study, written and successfully defended by Jan Havlíček, I believe it was concluded that blood group doesn’t impact performance.
After some time we returned to the original set of data and conducted a new, more meticulous analysis. It turned out that in the first analysis we made a novice error. In the time crunch (undergraduate theses are always completed at the last minute; it must be some natural law, or a perhaps the students were instructed to do so somewhere in the college handbook) we analyzed the effect of the Rh factor and toxoplasmosis on subject performance for each factor separately. But when we analyzed both factors together, we found that the effect of toxoplasmosis on performance is significantly different in Rh positive versus Rh negative people. Among Toxo negative people, the Rh negative subjects performed much better in the simple reaction time test, exhibiting much shorter reaction times than the Rh positives. Among the Toxo positive subjects, the results were drastically different: the Rh negative people performed much worse than did the Rh positives. While the Rh positives in the Toxo positive and negative groups performed about equally, the Toxoplasma-infected Rh positives and negatives were markedly different – indicating that after infection, the performance of an Rh negative person gets significantly worse.
We confirmed this surprising discovery on a number of subject groups, some of which numbered several thousand people, and saw that the joint effect of Toxoplasma and the Rh factor was usually very strong. These results were significant but unexpected, because to date none had uncovered the biological function of the Rh factor, nor how it could influence human behavior and physiological characteristics. It’s known that the Rh factor is a protein found on RBC membranes. In a significant part of the population, the respective gene has a large deletion – a large chunk of it is missing. A human, whose two copies of the gene have this deletion, is called Rh negative; there are no Rh proteins on his RBC membranes. If at least one copy of the gene does not have the deletion, then the person is Rh positive. Rh positives can be separated into homozygotes and heterozygotes. An Rh positive homozygote has both complete copies, whereas an Rh positive heterozygote has only one. The function of the protein on the RBC membranes is unknown; it seems to be a membrane pump, but it’s not clear whether it pumps ammonium ions or CO2 from one side of the membrane to the other (currently, CO2 seems to be the more likely option).
Until now there were no known biological manifestations of Rh negativity or Rh positivity. The only discovered manifestation of the two forms of the Rh gene was not related to the factor’s biological function. Over fifty years ago, it was discovered that when the child of an Rh negative woman and an Rh positive man gets the Rh positive allele from his father, then the mother becomes immunized against the Rh factor during the pregnancy or delivery. Her white blood cells (WBC) begin producing antibodies against the proteins on RBCs of Rh positive people, and these antibodies can harm the fetus, especially when the mother is carrying her second or latter Rh positive child. Usually, during the first pregnancy with an Rh positive child, the mother is just immunized; but in subsequent pregnancies, her immune system attacks Rh positive children more and more. Today, of course, doctors routinely screen for the risk of this so-called hemolytic disease of the newborn, and if a mother is Rh negative, then her child is treated immediately after birth. The Rh factor of the father does not influence the doctor’s decision to administer treatment – the risk that the alleged father is not actually the biological father is too great. To prevent the actual immunization, Rh negative mothers are given antibodies against Rh positive blood – these antibodies latch onto the RBCs of the child that made it into the bloodstream of the mother, so that the mother’s immune system never realizes their presence and doesn’t start producing its own antibodies (Box 69 Are women chimeras, and if so, what does it mean for them?).
|
Box 69 Are women chimeras, and if so, what does it mean for them?Chimeras are mythological creatures, composed of the parts of different creatures. Biologically, a chimera is an organism whose body consists of entire organs which are genetically distinct, while a microchimera contains only individual genetically separate cells. Strangler figs are found in the tropical rainforest; they use other trees as a climbing framework, and generally end up killing them (usually by completely blocking off their access to sunlight). At first and second glance, the strangler fig looks like a normal tropical species. But a genetic analysis of the tree shows that its body consists of genetically distinct segments. The strangler fig belongs to a group of species, whose seeds make their way onto tree branches in bird droppings. In order to grow into a tall, stately tree, a seedling must first let its roots crawl several meters from the treetop to anchor themselves in the ground. Meanwhile, the seedling is racing against time, and maybe even the stranger figs that sprouted from the seeds found in another pile of bird droppings. The group of seedlings which first takes root wins, and eventually strangles not only the tree that it’s climbing, but also its competitors – the other strangler figs. That is why it pays off for the seedlings in one pile of bird droppings to work together to form a single composite tree. Most humans are probably microchimeras, because occasionally during pregnancy some of the cells from the mother break into the embryo. Conversely, cells from the embryo also find their way into the mother. The embryonic cells carry genes from the father as well as the mother – and some of the gene variants from the father may not be found in the mother. This is of great value to the mother, because many of the escaped embryonic cells make their way into various tissues in the mother and begin to differentiate among each group of specialized cells. There have been cases in which a woman suffering from a genetic defect got significantly better after going through pregnancy, because cells from the embryo carrying copies of the functional gene from the father “fixed” parts of her body. It’s possible that this colonization of the mother organism by cells from the embryo developed because it was evolutionarily advantageous. For the child, it is clearly beneficial to fix its mother in time, thereby its chances of getting better and longer care once it is born. It’s even possible, that part of the reason women have a longer average lifespan than men, is because their bodies are rejuvenated during pregnancy by embryonic cells. Microchimerism, however, is not always beneficial for women. If a woman has had children with several different men, then her body contains various populations of genetically distinct cells, so her body must learn to tolerate multiple kinds of antigens. It has been suggested that this could be responsible for the deteriorated health of such women. |
Please excuse a brief digression. Among Rh negative women we found an unusually high prevalence of latent toxoplasmosis. It’s possible that when these women are preventatively given Rh positive antibodies, they also get Toxoplasma – or, they may only be getting antibodies against Toxoplasma along with the Rh antibodies. In the second case, the women would still show up as Toxo positive, because our diagnostic test is based on the presence of Toxoplasma antibodies. I sincerely hope that the second case is true; we should certainly determine that it isn’t the first. Infecting thousand of Rh negative mothers with toxoplasmosis during the preventative administration of antibodies, isn’t something that the doctors (and mothers) would be too happy about.
The existence of two forms of the human Rh gene was a great evolutionary mystery. It’s hard to imagine how this polymorphism could have developed, and primarily, how it can it can be sustained. Clearly, the carriers of the less common Rh gene type should be at a disadvantage in natural selection. Imagine that originally, everyone in a population was Rh negative, until there appeared one Rh positive mutant. The majority of the population was Rh negative, so an Rh positive male offspring of this mutant would usually reproduce with Rh negative women. Most of his children would die from the hemolytic disease of the newborn, so he’d leave behind fewer descendants. Natural selection would penalize carriers of the rare mutant gene, and according to evolutionary rules, it seems that sooner or later the Rh positive form should die out.
The same applies vice versa. Let’s imagine a population of only Rh positives, in which one individual were born an Rh negative mutant. Again, he and his offspring would be at a disadvantage to carriers of the original Rh positive gene form. In this case, the Rh negative women (with two copies of the mutant gene) would be disadvantaged, because they would usually reproduce with Rh positive men and some of their offspring would die of hemolytic anemia (Box 70 Genetic polymorphism: what it is, where does it come from and how can it sustain itself in nature?).
Box 70 Genetic polymorphism: what is it, where does it come from and how can it sustain itself in nature?Genetic polymorphism is the presence of different variants of the same gene (called alleles) in a species population. It is a nearly universal phenomenon. It’s nothing strange to find a population with one very frequent allele and several almost negligibly rare ones. New gene variants (alleles) are constantly created by mutations, and simultaneously eliminated by natural selection. Most new alleles are harmful, and carriers of a new gene variant are disadvantaged and die out. Occasionally a beneficial mutation will form, which affords its carriers greater biological fitness, so they leave behind more offspring than do the carriers of the original gene variant. As a result, the new beneficial allele grows to predominate in the population, and the genetic polymorphism returns to a low. It’s harder to explain why many genes have multiple variants which remain at comparable frequencies. Most often it’s the result of cyclical selection, frequency-dependent selection or selection which favors heterozygotes. Cyclical selection occurs due to cyclical changes in the environment, due to things like seasonal variation, so in different phases of the cycle, the carriers of different alleles are at an advantage. In frequency-dependent selection,an allele is harmful or beneficial depending on its prevalence in the population. For example, when two gene variants differ in terms of preferred diet, the less common variant will always be at an advantage, since it will have less competition for food. If gene variant A is less frequent in the population, then the respective food source A will be more available to variant A carriers – so they will be at an advantage. They’ll reproduce and have more offspring than variant B carriers, so their prevalence in the population will grow, and they’ll lose their advantage. The third possibility is selection for heterozygotes. Strictly speaking, it’s a type of frequency-dependent selection. Heterozygotes, with two different alleles, exhibit the greatest biological fitness; whereas homozygotes, with two copies of any one allele, are disadvantaged. As soon the frequency of a certain gene variant A dramatically decreases in the population, then allele A will be found almost exclusively in the heterozygotes (the chance that two rare allele A carriers will find each other and produce an allele A homozygote becomes very low). Since the heterozygotes have more offspring, the frequency of the now rare allele will begin to increase. As result, none of the gene variants can continually predominate – genetic polymorphism will always be maintained (see also Box 35 How I refuted Darwin).
|
A number of famous evolutionary biologists looked for a mechanism which would explain why two forms of the Rh gene occur throughout the human population, but without success (77) (78). Our data shows that the long-sought mechanism could be a greater resistance of Rh heterozygotes against the negative effects of toxoplasmosis. If there were no toxoplasmosis in the population, as might have been the case in Europe before the arrival of domestic cats, then Rh negative people would be advantaged, because they have shorter reaction times. But if toxoplasmosis were widespread in the population – as it likely was in Africa, where our species evolved – then the Rh positives would be advantaged, because their performance doesn’t worsen with toxoplasmosis. This could explain why Rh negatives are significantly less prevalent in Africa (and Asia) than in Europe.
Key to understanding the survival of both Rh alleles is the observation that Rh positive heterozygotes are more resistant to the harmful effects of toxoplasmosis (Fig. 38) (79). When we looked at the levels of Toxoplasma antibodies (which can be used to approximate when a person was infected) and reaction times of our test subjects, we found that the reaction times of Toxo positive Rh negatives worsened soon after infection. The performance of Rh positive heterozygotes does not worsen with time after infection – their reaction times do not correlate with the antibody levels. Rh positive homozygotes, with two functional alleles, slowly worsen with time after infection. The reaction times of the individuals with low antibody levels (long after infection) are about as bad as those of Rh negatives just after infection.
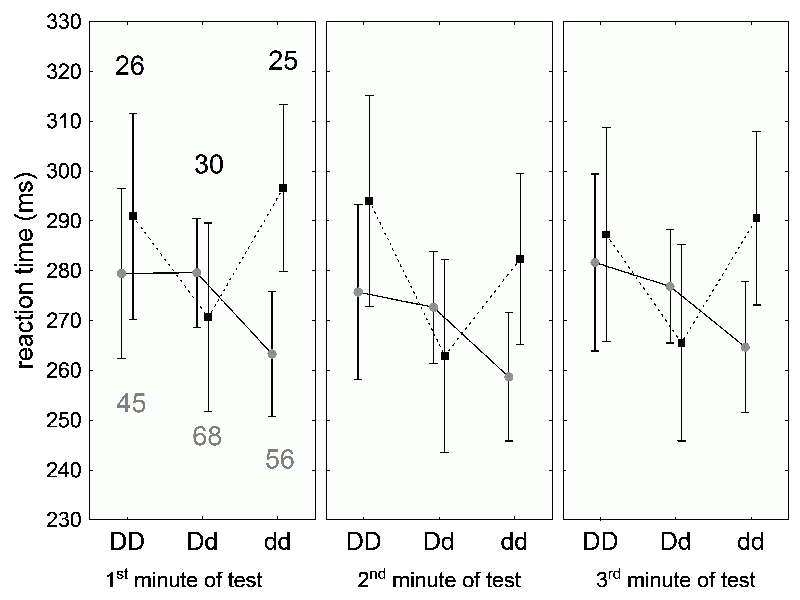
Fig. 38 The effect of the Rh factor and Toxoplasma infection on the reaction time of blood donors in three minute tests. Rh negative homozygotes (dd) have the best (shortest) reaction times when Toxo negative (gray circles). but the worst reaction times when Toxo positive (black squares). As for Rh positive heterozygotes (Dd), their Toxo negatives have good reaction times, but the Toxo positive times are actually better. Finally, Rh positive homozygotes (DD) have below average times when uninfected, and worse times when infected (with the exception of the second minute of the test) – though not as bad as those of infected Rh negatives. The numbers in the first graph indicate the number of Toxo negative (gray) and Toxo positive persons (black) in each groups.
The highest reaction time of the heterozygotes indicates that both forms of the Rh gene could remain in the population through the known mechanism of selection for heterozygotes (Box 70 Genetic polymorphism: what it is, where does it come from and how can it sustain itself in nature?). This is the same mechanism which maintains the gene for sickle-cell anemia. This gene codes for a special form of hemoglobin, called hemoglobin S, which becomes fatal for its carrier when that person has two copies (which makes him a hemoglobin S homozygote). Children who inherited this allele from both their parents are born with deformed sickle-shaped blood cells, and die of sickle-cell anemia. If the child is a heterozygote, and carries only one copy of the hemoglobin S gene (and the other copy is normal), then it suffers from a much milder form of the disease. Of course, even this milder form is disadvantageous for the individual. But in places with malaria, the presence of this deformed hemoglobin gives the heterozygote the ability to tolerate the disease. Malaria is a serious health factor – in some parts of Africa, many children die from it. So it’s not surprising that the geographic distribution of malaria matches up with that of the gene for sickle-cell anemia.
Our hypothesis is that a similar heterozygote advantage effect occurs in the case of Rh positivity; instead of malaria, the disease is latent toxoplasmosis and the associated worsened reaction time (possibly along with other, still unknown effects). As I mentioned, this mechanism could explain not only the old evolutionary mystery of the two Rh gene forms, but also the low frequency of Rh negativity in Africa, where, probably even today, toxoplasmosis occurred in about 90-100% of village inhabitants in some regions. Furthermore, it would explain the spread of the allele for Rh negativity throughout Europe, where, until “recently,” there were no felids in close proximity to humans, and the prevalence of toxoplasmosis was likewise very low (Box 71 Why are Rh heterozygotes better off, and what if they actually aren’t?
Box 71 Why are Rh heterozygotes better off, and what if they actually aren’t?In the case of the Rh factor, and the resistance it can give against latent toxoplasmosis, it’s not easy to explain why the heterozygotes should be better off. For other genes, the situation is simpler. Each variant of the gene has a different function or works optimally under different conditions. Heterozygotes take advantage of having both variants of the gene, and both functions available. The situation with the Rh factor is different because the Rh negative form isn’t actually a variant of the gene – the deletion is so large, that the protein doesn’t get synthesized. Whereas Rh negatives have no Rh proteins on their RBCs, Rh positive heterozygotes just have less of them than do Rh positive homozygotes. But if having fewer Rh proteins were an evolutionary advantage, then natural selection would achieve it more easily changing regulatory elements of the respective gene (and then the advantage could be enjoyed by all members of the population, not just heterozygotes). There are several possible explanations for the apparent heterozygote advantage regarding the Rh factor. It’s known that the Rh protein occurs on the surfaces of RBCs in a complex with several other proteins. In Rh negative people, these protein complexes also exist, but the missing Rh protein is replaced by a similar protein coded by a different gene. It’s possible that the two types of complexes (with and without the Rh protein) serve different functions, and so it benefits the heterozygote to have cells of both types. But there is also a completely different explanation. Rh positive heterozygotes and homozygotes aren’t usually differentiated among blood donors. One would have to use expensive molecular biological methods, instead of the commonly used serological techniques. So in our study, we didn’t definitively know the Rh positive homozygotes from the heterozygotes. But we were able to estimate it in another way. We took advantage of the existence of a so-called linkage disequilibrium. Genes found close to the Rh gene have several possible variants (alleles), and certain combinations of these alleles are almost always associated with the Rh+ allele, and others with the Rh allele. So the advantage exhibited by the Rh positive heterozygotes could actually be due to the genes we used to estimate whether the person was a heterozygote or a homozygote. In the future, we’ll have to repeat our study and distinguish between the homozygotes and heterozygotes using direct methods of molecular biology. It’d be funny to find that there was no Rh gene heterozygote advantage after all, and that it was our improvised, “incorrect” method that led to the interesting discovery. (But it might, with a little luck, lead to the discovery that other alleles of the neighboring genes are associated with the observed heterozygote advantage).
|
When we found that the effect of latent toxoplasmosis on the human organism depends on that person’s Rh factor, we re-evaluated our previous data. Whenever it was available, we added each person’s Rh factor as another variable in the statistical models. We found that in many cases there was interaction between the Rh factor and toxoplasmosis, meaning that toxoplasmosis manifested differently in Rh positives than in Rh negatives (79) (80).
An interesting result came from our long-term study, in which we observed the effect of toxoplasmosis on the risk of accidents among military drivers (32). Our coworkers tried to convince all the military drivers who were examined by the Psychology Department of the Central Military Hospital to agree to toxoplasmosis screening. When they finished their mandatory military service, about 12-18 months later, we examined the files and records of the military police to see which of drivers had a driving accident; and then looked to see if the risk differed between the Toxo positives and negatives. The set-up of this study was much better than the set-up we used for our first studies on the risk of car accidents among Prague inhabitants. The first studies had a basic set-up, and would be categorized as case-control studies. This means we looked at the prevalence of toxoplasmosis in two groups: in the drivers who had a driving accident, and in a control group of people without accident who lived in the same area. But this makes interpreting results difficult. We couldn’t determine whether the differences in toxoplasmosis prevalence between these two groups, were because the drivers who crashed were more likely to be Toxo positive, or because the control group was less likely to be Toxo positive, compared to general population. The control group was created when family physicians asked their patients if they wanted to be included a serological survey – if their blood could be tested for the presence of antibodies against a number of pathogens. If Toxo positive people were less likely to consent, then our control group would have a falsely low prevalence of toxoplasmosis. As a result, we would incorrectly determine that Toxoplasma raises the risk of driving accidents, when it was really just lowering the chances that a Toxo positive would get into our control group.
The set-up we used with the military drivers is called a prospective cohort study. This study did not have the ambiguity of the case-control study, because it was carried out only on the drivers who were willing to participate in it. This ended up being practically all of the military drivers, because over 70% agreed to participate. We tested all of these soldiers for latent toxoplasmosis and their Rh factor; subsequently we determined whether or not they’d crashed during their mandatory military service. The increased risk of driving accidents among Toxo positive drivers had to mean that toxoplasmosis affected the probability of an accident; rather just influencing an individual’s willingness to participate in the study (Box 72 You can’t expect miracles, even from a prospective cohort study).
Box 72 You can’t expect miracles, even from a prospective cohort studyUsing our prospective cohort study we were able to decide between the two hypotheses which could explain the results of our original case-control study – we found that Toxoplasma raises the risk of a driving accident, rather than lowers a person’s chances of being in our control group. But even in the prospective cohort study, we didn’t rule out the possibility that a third unknown factor could be responsible for the correlation between toxoplasmosis and driving accidents. For example, it’s possible that careless individuals are more likely to get into driving accidents and get infected by a parasite. Or it’s possible that driver’s from villages don’t have experience with city traffic and are more likely to get into accidents during mandatory military service. People who grew up in the country are also more likely to be infected by Toxoplasma . Of course, to explain the statistical correlation between toxoplasmosis and driving accidents using one of these hypotheses, we’d first also to explain why the correlation is true for only Rh negative people. Not that we couldn’t do it – it would just be difficult. Such a hypothesis would have to be fairly complicated, and include additional assumptions: for example, that more Rh negative people come from villages than towns. A simpler and more reasonable explanation would be that toxoplasmosis increases the risk of driving accidents in Rh negative people – especially when we consider that in independent studies we already proved that toxoplasmosis worsens reaction time in Rh negatives. Statistics is a powerful tool, but we can’t get far without common sense.
|
We started our study before knowing about the possible role of the Rh factor. The study ran around five years, and at first it seemed that it would bring negative results. Simple tests showed no statistically significant difference between Toxo positives and negatives in the risk of car accidents. But as soon as we included the variable of the Rh factor in our analysis, we got data which agreed with results from our previous performance testing of Rh positive Toxo positive people. In Rh positives, infection by Toxoplasma had practically no effect on the probability of driving accidents, whereas in Rh negative subjects it greatly increased this risk. In Rh negatives, the risk of a driving accident decreased with lower Toxoplasma antibody levels. Those with the highest antibody levels, who had been infected fairly recently, had a 17% probability of a driving accident – about six times greater than that of uninfected Rh negatives or Rh positives (both infected and uninfected) (Fig. 39). That corresponded with the results of the previous study, because even among Prague inhabitants the highest risk of driving accidents was among people with relatively high antibody levels. Once more we found that although reaction time worsens with time after infection, the infected drivers are gradually able to adapt to their worsened abilities. Our study brought another
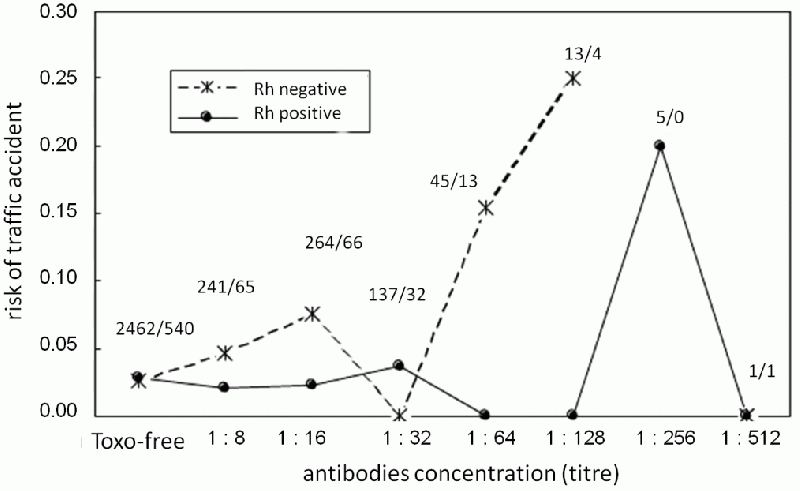
Fig. 39 The relationship of traffic accident risk and the titer of Toxo-specific antibodies in Rh positive (circles) and negative (crosses) military drivers. The uninfected drivers have a similar risk of a driving accident, regardless their Rh factor, but Toxo positive Rh negatives have a higher risk, which further increases with increasing levels of Toxoplasma antibodies. (The antibodies were determined using complement-fixation test.) Rh negative drivers with a titer of 1:64 – individuals which were probably infected not long ago – have a six times higher (17%) probability of an accident during their military service than do uninfected drivers. The numbers indicate the ratio of Rh positive to Rh negative drivers with a given titer of antibodies.
result, which we probably could have expected. A suspiciously large number of the drivers who had a driving accident had not participated in our study. This indicates that the drivers with the greatest risk of driving accidents refused to participate in our testing. Unfortunately, we cannot publish this result in a scientific journal, because we don’t have the informed consent of these “uncooperative” drivers. But it shows that a person’s chances of a driving accident are influenced not only by his driving abilities, but also by his personality traits. Such as his willingness to cooperate.
The case of the Rh factor and toxoplasmosis can be used as an example of an often-recounted mistake of scientists. The error probably comes from a time when sophisticated statistical methods weren’t commonly available. In the 80s, when I was studying at the Prague College of Natural Sciences, my mentor Jiří Čerkasov tried to (fortunately unsuccessfully) drive home the rule: one factor, one experiment. In other words, a study should supposedly be set-up so that each experiment analyzes the effect of one factor. But if we had followed taken this approach, we never would have discovered the interaction between the Rh factor and toxoplasmosis. When we studied the effect of the Rh factor on reaction times for a group of Toxo positive and negative people, as undoubtedly many scientists had done before us, we found no difference in reaction time between Rh positive and negative people. But when we included both the factors in our analysis (toxoplasmosis and the Rh factor), we suddenly discovered that uninfected Rh negative subjects have better reaction times than do uninfected Rh positives – but infected Rh negatives have much worse reaction times do Rh positives. The same problem would have happened in our first studies on Toxoplasma’s effect on the human psyche, if we had analyzed the men and women as one group. Since toxoplasmosis often shifted the same Cattell’s factor in opposite directions for men versus women, we never would have discovered the effect of the parasite without taking gender into account.
In my experience, any study requires one to look for and then simultaneously analyze as many factors as possible. To this end, I always tried to track several variables and run the same people through the maximum number of tests. Then I could analyze a large number of relationships and interactions between the studied variables. Of course, this approach also has its bad parts. As we study more relationships, there is a greater risk of Type I errors – there is a greater probability that some of the relationships we observe between the variables will happenstance. As I like to stress, the p-value given by a statistical significance test (which helps us to estimate the probability that an observed phenomenon is the result of chance), can only be applied to a single statistical test. If we carry out 20 statistical tests, it’s very likely that one of them will turn out as statistically significant just due to chance.
I believe that it’s definitely worth it to run the risk. But you have to know how to deal with the results of such experiments. I teach my students to treat them like the results of an exploratory study (see Box 10 Statistical evaluation of data). You always have to meticulously explore the data from all possible angles and find as many potentially interesting phenomena as possible. It’s not advisable to rely on correction for multiple tests, e.g. such as the Bonferroni correction; in other words, the kind of technique usually recommended by most of statisticians when carrying out several independent tests. It’s much more useful to test an observed phenomenon on another, independent group. In a way, each of my studies is both confirmatory and exploratory. They’re confirmatory because I try to confirm the existence of phenomena seen in previous studies or based on theories created by myself or other scientists. They are also exploratory because I always look for other possible phenomena in the new data, whether they relate to the original direction of our study, or are just relationships between variables that we happened to include. Like I said, we usually test a large number of variables – both to statistically filter out their confounding effects on the target variable, and to discover any possible relationship between the confounding variables (see also Box 20 Dependent, independent, and confounding variables; fixed and random factors). When we first discover a new effect or an interaction between two variables, we don’t put too much stock in it – until we find the same effect or interaction in a different group (or preferably, several groups). The smaller our data sample, the more false positive results we usually obtain. For this reason, we don’t generally publish results obtained on one test group – even if the results were very interesting. But if we confirm them on other groups, we do try to publish the results, whether they relate to toxoplasmosis, or the relationship between sexual preference and the length ratio of fingers on the left hand (Box 73 Do homosexuals have a longer pointer finger than ring finger?) As a result, a person not familiar with our work might look at our published articles and be hard-pressed to determine our field of study.
Box 73 Do homosexuals have a longer pointer finger than ring finger?It depends. All relationships between the length ratio of these fingers and psychical or biological traits are purely statistical. So the length ratio of the fingers cannot be used to determine whether someone is a homosexual or heterosexual, or even if they’re a man or woman. Nevertheless, if we have a group of 30 men and 30 women, then according to the length ratio of the 2nd and 4th fingers, we’ll easily tell the gender groups apart. In the case of sexual orientation, it’s a bit more complicated. Among women, the difference between homosexuals and heterosexuals generally does not correlate with the finger length ratio. On the other hand, a difference in the length ratio for homosexual versus heterosexual men is usually observed in both Americans and Europeans (81) (82) (83). The problem is, whereas among Europeans homosexual men have a lower length ratio than heterosexual men (and men identifying themselves as bisexual have an even lower length ratio), among Americans it is the other way around. We have not yet found an explanation. In one study conducted on visitors of the Municipal Library of Prague, we measured the length of each finger, and administered an anonymous survey to determine sexual preference and behavior. The group had about 1000 people, but only between 800 and 900 (roughly same amount of men as women) answered the questions regarding sexuality. Our results showed no difference in finger length ratio between homosexuals versus heterosexuals, neither among men, nor among women. Surprisingly, we found a significantly higher length ratio (a relatively longer pointer finger) among women who answered that they were sexually aroused by tying hands (whether their partner tied their hands, or they did so their partner), i.e. moderately sadomasochistic practices. On a 7-point scale, 56% of women and 46% of men put a 4 or higher for how much they were aroused by their partner tying their hands. As for tying their partner’s hands, the same percentage of men and women (44 and 45, respectively) were very aroused, choosing 4 or higher on the 7-point scale.
|
After we started including the effect of the Rh factor in our models, we saw that the effect of Toxoplasma, or rather the interaction of Toxoplasma and Rh factor, showed up not just in the results of simple reaction time tests, but also in those of more complex performance tests. Usually the interaction manifested similarly as it did in the simpler tests. Rh negatives performed better than did Rh positives, but only among Toxo negatives. When the people were infected by Toxoplasma, then the Rh negatives performed significantly worse. But in some cases this wasn’t true. For example, we found that Rh negative Toxo positive men performed better in intelligence tests and have higher IQ values, than did uninfected Rh negatives. But among Rh positive men, we saw no such difference. We haven’t figured out why the effect of the Rh factor and toxoplasmosis was different on intelligence than on reaction time. It’s possible that Toxo positive Rh negative people have slower reaction times, but are more careful, so they are better at solving certain tasks. In this case, infection by Toxoplasma might have an effect opposite to what we observed for simple reaction times.
Our next results, obtained as a side-product of a primarily Toxoplasma study, indirectly supported this possibility. When we graphed reaction time versus intelligence for a group of soldiers, we found that the shorter the reaction time, the worse the result of the IQ test. But this was only true for tests which measure nonverbal intelligence. Tests which targeted verbal intelligences did not show such a relationship.
Since I already mentioned the results of the intelligence tests, I should probably admit that even after 20 years of research, we aren’t sure if toxoplasmosis plays a role in intelligence. In our first studies, it seemed that the intelligence of infected men was lower than that of uninfected men. In some groups, the difference between the average intelligence of infected vs. uninfected men was statistically significant. For women, some groups showed an opposite relationship – that intelligence of infected women was higher than that of uninfected women. But in such tests it’s always hard to differentiate between the direct and indirect effects of toxoplasmosis. For example, we can’t determine whether Toxo positive women perform better in intelligence tests because they’re really smarter, or because they try harder when completing the test. As I mentioned, infected women have a higher superego and are also more sociable (Cattell's factors G and A), which are certainly personality factors which can make someone try harder on the test. Furthermore, the intelligence of Toxo positive versus Toxo negative people can be different without toxoplasmosis having any effect – direct or indirect – on intelligence. First off, the cause-effect relationship may be the other way around. It’s possible that more intelligent people are for some reason more likely to get infected than are less intelligent people. And secondly, there may exist an unknown, third factor which influences both intelligence and the risk of Toxoplasma-infection. Our results indicate that this third factor could be the person’s resident population, or rather the difference between a city versus country life-style. A number of our subject groups, including the groups of soldiers, have data on the size of each person’s city, town or village. When we looked at the size of a person’s hometown versus his nonverbal intelligence (measured using the so-called VMT questionnaire), we found no statistically significant relationship. But when we examined the same thing for verbal intelligence (measured using the Otis questionnaire), we discovered that people from larger towns tested as more intelligent. Moreover, it’s very clear that people living in villages have a much higher probability of catching Toxoplasma than do people from larger towns or cities. When we included the factor of residence (settlement population) in our models studying the relationship of toxoplasmosis and verbal intelligence – or when we looked at the relationship separately for people from villages, towns and cities – the correlation between Toxo-positivity and verbal intelligence was no longer statistically significant (Fig. 40). Currently, we are starting on a project in which we will meticulously study the relationship between latent
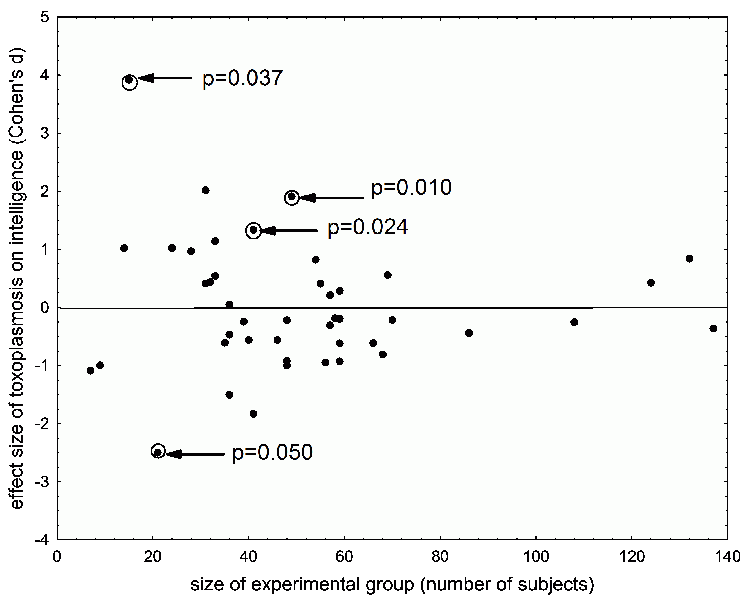
Fig. 40 A study of the relationship between Toxoplasma infection and intelligence. Over the course of 5 years, we examined 42 groups (with 10-136 of soldiers in each group). Only in four groups were the differences between infected and uninfected soldiers in verbal intelligence, as determined by Otis test, statistically significant after filtering out the size of their settlement. The funnel plot (wide end to left) in which the size of observed effect is plotted against the number of individuals in the study demonstrates that the effect of Toxoplasma was significant only in small or medium size studies, and that all individual points representing the effect are located symmetrically around the null line. Therefore, Toxoplasma infection doesn’t influence the verbal component of intelligence. (In other words, if you believe my writing to be nonsensical or stupid, I regret to say that I can’t blame it on Toxoplasma.) If this were a real meta-analysis (which funnel plots are commonly used for), and the individual points indicated the results of already published studies, then the symmetry of graph would testify that there is not a substantial publication bias in the specific research field. If there were publication bias, then values would be missing on part of the funnel plot’s wide margin (on our graph, the values on the left) – the small controversial studies which results weren’t in agreement with mainstream opinion about direction of observed effect would not be published. In small studies it is certainly possible to prove, for example, that Toxoplasma significantly increases or decreases intelligence. However, if most scientists believe that toxoplasmosis decreases intelligence, then reviewers or “auto-censorship” will not allow opposite results from a small studies to be published. A funnel plot is usually hollow on the wide end (in this case, on the left, close to the null line) – studies which measure a small effect are usually published only when conducted on a large subject group.
toxoplasmosis and intelligence. In this study, we will use a complex IQ test that measures a number of independent components of intelligence. I’m very curious to see the results – I find it hard to believe that Toxoplasma could influence several psychological factors but not tamper with intelligence.
Another study showed that the Rh factor significantly influences how an individual’s personality type changes after infection (84). For a group of blood donors, we measured reaction time and then asked the subjects to fill out Cloninger’s and Cattell’s psychological questionnaires at home. By mail, we received 302 questionnaires from 213 men and 89 women. About the same percent of men and women sent back the questionnaires – it’s just that there are usually more male blood donors. Once again, we confirmed that Toxo positive people have significantly lower Cloninger’s factors of NS (novelty seeking) and ST (self transcendence). Infected people also had a higher Cattell’s factor of Superego Strength (G) and a lower Cattell’s factor of Vigilance (L) – this was true for both genders. We also found a higher factor N (privateness) among the Toxo positives of both genders. Because we included the Rh factor in the analysis, we could distinguish how changes in psychological traits depend on this factor. For example, we discovered that the Rh factor influences Cloninger’s factors of HA (harm avoidance) and CO (cooperativeness), which are lower and higher, respectively, in Rh positives. It also influences Cattell’s factors of C (Emotional Stability) and L (Vigilance), which are higher and lower, respectively, in Rh positives. Moreover, we found that toxoplasmosis influences Rh positives differently than Rh negatives, for the following factors: Cloninger’s RD (reward dependence) and CO (cooperativeness); and Cattell’s C (Emotional Stability), M (Abstractedness) and Q4 (Tension). For example, infected Rh negatives have lower cooperativeness (CO) and lower Emotional Stability (C) than uninfected Rh negatives, whereas it’s the opposite way for Rh positives. We used the same data from the blood donors, and tried what would happen if we didn’t consider the effect of toxoplasmosis in the analysis. It turned out that when we don’t filter the effect of toxoplasmosis, the Rh factor has no statistically significant effect. Out of the seven Cloninger’s and sixteen Cattell’s factors, the only one that differed between the Rh positives and Rh negatives was Vigilance; after a Bonferroni correction for multiple tests, even this difference became statistically insignificant. Similarly, when we analyzed the same data for Toxo positivity without filtering out the effect of the Rh factor, we found statistically significant differences in only five psychological factors. Since nobody before us studying the effect of the Rh factor on personality included the effect of toxoplasmosis in their analyses (and why would they think to do so?), there were no publications indicating that the Rh factor could influence the human psyche. In the 70s, the great Raymond B. Cattell himself published a study describing the effect of the Rh factor on the human psyche (85), but even he wasn’t taken seriously (I was tempted to write “he” with a capital H, but the editor would have probably changed it anyway). The problem was also that most authors focused on the possible effect of blood groups according to the ABO system, and neglected the Rh factor. It might be because the staunchest believers in the influence of blood groups on the psyche are the Japanese, who, like most Asians, are almost all Rh positives. Of course, after filtering out the effects of Toxoplasma and the Rh factor, I also examined our data to see if ABO groups had an effect on the human psyche. They don’t. So the Japanese can keep trying, but they probably won’t find any such effect. But that doesn’t stop them from firmly believing in it (Box 74 Why it’s a shame that the Japanese and Australian aborigines are Rh positive).
It’s important to wonder how specific the protective effect of Rh positivity is. Does it protect only against the effects of latent toxoplasmosis, or does it impact other factors? Our results so far indicate that the second option could be correct. In our study on the effects of the Rh factor and toxoplasmosis on the human psyche, we noted that the effect of
Box 74 Why it’s a shame that the Japanese and Australian aborigines are Rh positiveFor the Japanese, the answer is simple – if almost all of them weren’t Rh positive, then they could easily repeat our study, and we would become famous (at least in Japan). I spent over a year at the University of Tokyo, and can say that the talk at every good party eventually turns to blood groups and their effect on intelligence and the psyche in general. If you’re bleeding to death in Japan and the doctor asks for your blood group, whatever you do, don’t say that you don’t know. The doctor might give you an understanding wink and immediately give you a transfusion of blood group B (I am just joking, of course, but only a little). The Japanese believe that people with the blood groups A or AB are more intelligent and generally more capable than those with blood group B, so when someone claims to not know their blood group, it’s clearly because they belong to blood group B. And why is it a shame that the Australian aborigines are Rh positive? Because in Australia, before the arrival of Europeans, there were no felids, and hence no toxoplasmosis. If Rh negativity became widespread in Europe until the arrival of domestic cats, and presumably, we might expect that this would apply even more to Australia. Oh well. Nature sometimes won’t listen to the advice of the evolutionary parasitologist, and decides matters for herself. In any case, it possible that a different gene variant in Australians, carries out the function provided by the Rh- allele so widespread in Europe. |
age on certain psychical factors differs depending on Rh positivity vs. Rh negativity (34). Our results showed that in blood donors, Dominance (factor E) significantly decreases with age, whereas Privateness (factor N) increases in infected people. A more detailed analysis showed that this relationship exists only in Rh positives (Fig. 41). It’s an interesting and unexpected result, because in the case of reaction times and in the case of weight gain during pregnancy (see the next chapter), it is the other way around: the Rh positives are actually protected against the negative effects of Toxoplasma.
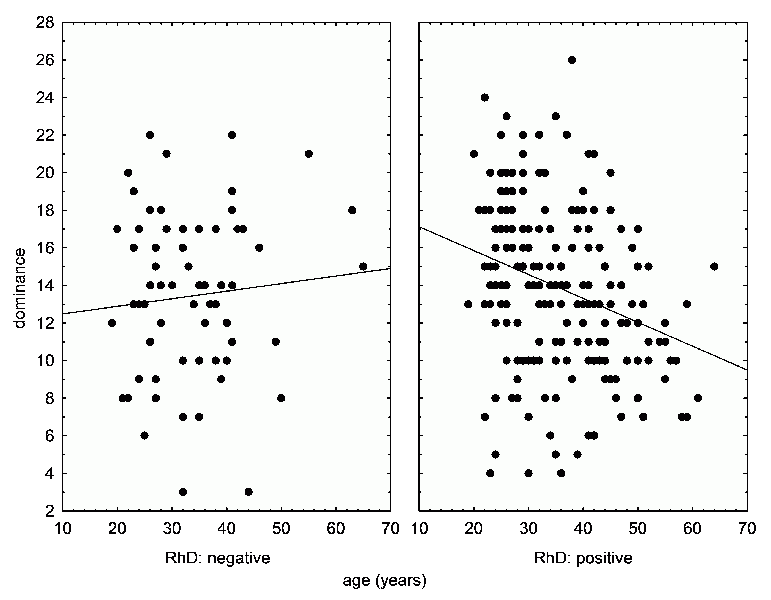

Fig. 41 The effect of the Rh factor on the correlation between the age of blood donors, and Cattell’s factor N (Shrewdness) (top graphs) and E (dominance) (bottom graphs). The graphs demonstrate that both psychological factors correlate with age only in Rh positive persons. In both cases, the correlation is statistically significant.
