I. How I didn’t become an immunologist nor a molecular taxonomist
How did it all begin? Quite differently than I tell the reporters, who come sniffing through the laboratories each year when silly season rolls around. They’re hunting for stories on how scientists battle with nature, day and night, to wrest away her closely guarded secrets. Dear readers, know this: It’s not true that “it all started when I joined the department of parasitology in the 90s, and cast around for a research topic that might encompass both parasitology and evolutionary biology.” Period. Don’t listen to journalists – they’ll believe and publish anything.
I’d say that the reality was much more interesting. After about four years working in the Department of Immunology of the Institute of Molecular Genetics of Czechoslovak Academy of Sciences, I returned to the Department of Parasitology at Charles University. My primary goal was to form a laboratory of molecular biology, and to dedicate myself to molecular phylogenetics – in other words, to continue with the topics I had researched before leaving the University. Most of all, I was determined to avoid immunology, though Jaroslav Kulda, the department head at the time, had originally invited me to his workplace to study this topic. Since I arrived in the department with my own salary fund, I didn’t feel tied to my bosses’ expectations (however, I always tend to play by my own rules, so the salary fund probably had little to do with it) (Box 1 How to arrive in the department with your own funds).
Maybe it wasn’t very nice or responsible of me, but I think that I finally gave the head of the department a sufficiently convincing explanation, for why I wanted to form a molecular biology instead of immunology lab (neither of these existed in the department at that time). I told him, “Immunology is an interesting scientific discipline, but under the conditions of the department, which has no history of immunological research, it could not be conducted at a decent level.” Not only did we lack the basic equipment, but we also were missing contacts with top international workaces, as well as sources for literature, chemicals– everything you can think of. Furthermore, we lack
|
Box 1 How to arrive in the department with your own funds for salaries This story reminds me of that common scenario in fairytales: kind-hearted Jack, on his way to save an enchanted princess, meets a hungry, weary traveler on the road. Out of the goodness of his heart, Jack shares his last piece of food with the traveler. The weary traveler then turns out to be a powerful sorcerer, who had taken this disguise to test Jack, and now rewards him for his altruistic behavior. About a month after the Velvet Revolution, with the country in disarray, several of us former members of the College of Natural Sciences arranged a meeting. We thought of returning to Charles University and establishing a new workplace, which would study theoretical and evolutionary biology. One of those present was Zdeněk Neubauer, who had been pressured to leave the University in the 80s; with his unorthodox opinions (and this is a very moderate term), he infuriated his more conformist colleagues like a red Soviet flag does an exceptionally irritable Western-oriented bull. At the end of the meeting, Zdeněk asked each and every one of us, whether we really wanted him on the team. I told him that while I usually disagree with his opinions, I’d be happy for him to be part of our project. At the time, I had no inkling that Zdeněk Neubauer was a good friend of both future president Václav Havel, and of Radim Palouš, the first president of Charles University after the revolution. In the stormy post-revolutionary period, Neubauer had a say in what happened with the salary funds of the former Marxism-Leninism department. Unlike Neubauer, I had no way of knowing that he would be the one to select employees for his Department of Philosophy and Natural history. After ensuring salary funds for most of his conspirators (evidently, not everyone passed the weary traveler test), he told us that we could stay at his new department, or were free to transfer to a department of our choice, along with salary funds. I was among the people who chose the second option (though only transiently, as it turned out). |
experience: in the Institute of Molecular Genetics, I primarily cloned interleukin genes, an activity closer to molecular engineering than to real immunology. Not even my fourteen months in the Department of Immunology at the University of Tokyo made up for my lack of experience in the field. Rather, they showed me that immunological research is difficult to carry out under the conditions of our University’s Department of Parasitology. I continued, “The most I could do here was assistant work, like preparing antibodies for the experiments of other colleagues, developing a diagnostic system to detect parasites, or look for a way to monitor the immune response of an infected host.” (Saying this, I got the feeling that something flickered in the eyes of the department head. It might have been that proverbial spark of hope, or just a trick of the light.) “But spending my time running a service laboratory was the last thing I wanted to do in a new, or really any, workplace.” (The light in the eyes of the department head dimmed). If I remember correctly, Jaroslav Kulda never reproached me for moving to molecular biology and then evolutionary parasitology, and made peace with the situation.
|
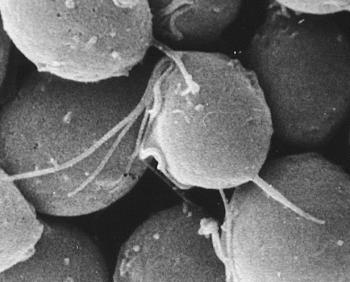
Fig. 2 The protozoan Trichomonas. A sexually transmitted parasite that was the subject of my undergraduate and later graduate study. Scanning electron microscope picture by Pavol Demeš. |
|||||||||
|
In contrast, we at the University had significantly better equipment and practical experience for studying molecular biology. In my undergraduate work, I had looked for DNA in the organelles of the parasitic protozoan trichomonad (Fig. 2). The search was unsuccessful, which might have had something to do with the fact that the particular organelles (hydrogenosomes) in this protozoan have no DNA, as was discovered many years later (notice how carefully the scientist formulates his conclusions). In the ancestors of trichomonads, all the hydrogenosome genes moved into the cell nucleus (see Box 2 Why some cellular organelles have their own DNA, while others, like hydrogenosomes, don’ t
Partly thanks to this, my undergraduate work acquainted me with a variety of interesting techniques from molecular biology, which I used in an effort to find DNA in this trichomonad organelle. I should mention that I selected the topic of my undergraduate work myself, despite emphatic warnings from my mentor Jiří Čerkasov, as well as his remarkable wife and co-worker, Apolena Čerkasová. Instead of organelle DNA, I finally discovered the first dsRNA virus in a protozoan cell, as well as the presence of repetitive DNA in the Trichomonas nucleus. My graduate work included further study of this virus in different strains of trichomonads. Unfortunately we published the discovery of dsRNA in Trichomonas, along with the subsequent proof of virion particles, later than our American competitors, but at least it was in a significantly worse journal However, in those days that was our workplace tradition. (I sometimes say “we” when discussing the Trichonomas experiments – not as a majestic plural – but rather to express the fact that experiments performed by undergraduate and graduate students are usually financed, planned, or co-planned by the student advisor; and usually several lab members are involved in the study, including other students and lab technicians.) Nevertheless, this time our American competitors didn’t have to try as hard. Unlike me, they didn’t have to drop their research for a year to become a tank platoon commander. Instead of running nucleic acids across an electrophoretic gel in the lab, I found myself, running four tanks and fifteen mischievous scamps across the snow-covered plains on a military base in the Doupov Mountains. I apologize to Captain Šic, the proud head of my battalion – who was famous for saying that hell would freeze over before any of us bean-sprouts (meaning us university types) would get an officer’s star, and yet probably spared me from future military call-backs (I suspect that he scribbled in his report: “Arm Flegr only if all has been lost!”) – again, I hope Captain Šic will forgive me when I say that my American competitors didn’t miss much in military service. The landscape of the Doupov Mountains might look nice from the vantage point of a horse-back rider, but certainly not to the shivering soldier sitting on the steel seat of a T-55 tank. After returning from the mandatory military service, we struggled to catch up to the Americans. Sometimes, in our lab experiments, we even closed the gap, but when it got to the final sprint – getting our work published – we usually came in second place. Even to this day, there are several interesting discoveries about Trichomonas dsRNA viruses that we haven’t yet published (Box 3 Trichonomas double-stranded RNA).
But the study of RNA viruses in protozoans wasn’t supposed to be the main research topic in my new laboratory. Already during my post-graduate study, I was well aware that our workplace had two major advantages over labs in other countries. In our department, we had a respectable collection of parasitic protozoans preserved in liquid nitrogen. And outside the liquid nitrogen, we had an equally respectable collection of parasitologists, who, in the quiet sanctuary of the Prague College of Natural Sciences (which, as my colleague Stanislav Komárek like to say, is the last traditional German university in the world), waited out the tumultuous evolution of international biology in the second half of the 20th century. By the start of the 80s, in most Western universities, the departments of parasitology were either dissolved or gradually colonized by molecular biologists and biochemists, who knew little about the parasites they used in their studies. In our university, up to the late 90s, most of the scientists in the department of parasitology, and even most of its graduates, were able to find parasites in nature, determine their species, isolate, and, when technically possible, use them to establish a lab culture. And these abilities can be extremely useful. For example, they served me well in the field of molecular phylogenetics, which I began studying sometime in the mid-80s. In the 90s, scientists had not yet determined the zoological or botanical classification of many unusual groups of protozoa. So it seemed that we had a great chance to use our natural advantages, with the aid of modern molecular biology techniques, to fill in the empty spaces of the taxonomic system. This proved to true, as over the years my molecular phylogenetics research team (Box 4 Research teams) gradually discovered the phylogenetic positions of several interesting groups of parasitic protozoans (5);(6). Other groups of parasites were classified more quickly by our competitors, be it by a couple months or couple years (7) (8), but that’s life. So how was it that I left my lab of molecular phylogenetics to study Toxoplasma’s effect on host behavior – in other words, to study the manipulation hypothesis? Simply put, it was happenstance. When I arrived in the college, I knew very little about the manipulation hypothesis, and I certainly didn’t expect to study it. In truth, I wasn’t all that interested in it. The manipulation hypothesis states that several parasites alter the behavior of hosts in an effort to spread from the infected host
into a new, uninfected host. For example, a parasite, in order to complete its life-cycle, might need its current host to get eaten by a certain predator (so that the parasite can spread to the predator). The parasite often is able to alter the behavior of its current host, so it is more likely to get caught and eaten. For example, the infected host may be less careful, more noticeable, spend more time in open space, or react more slowly when in danger (Fig. 5). When I arrived in the department, most of what I knew about the manipulation hypothesis came from my favorite books, The Selfish Gene and The Extended Phenotype by Richard Dawkins (see Box 5 The Selfish Gene). Among other things, Dawkins discusses the case of the Lancet liver fluke Dicrocoelium dendriticum. During its life-cycle, this fluke needs to get from its intermediate host, an ant, into one of its definitive hosts, such as a sheep. Now, sheep don’t usually eat ants, but the fluke has a very elegant fix for this minor detail. It reprograms the behavior of its host, so that come
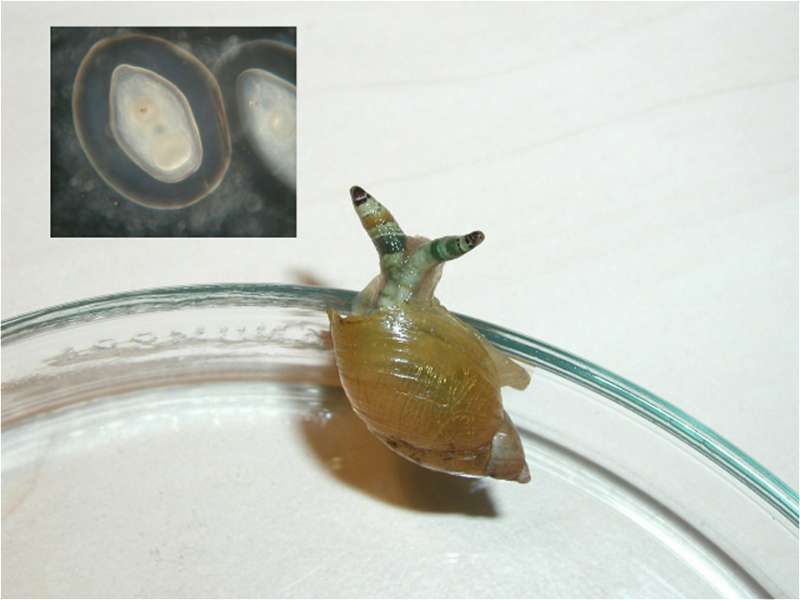
Fig. 5 The green-banded broodsac (Urogonimus macrostomus, originally called Leucochloridium macrostomum or Leucochloridium paradoxum), residing in an amber snail (Succinea putris). You can see the large, colorful and swollen broodsacs that this parasitic flatform has created on the snail. During the day, the broodsacs move to the snails’ tentacles and cause them to pulse with light. Insect-eating birds (the definitive hosts of the green-banded broodsac) confuse the tentacles with caterpillars and eat the snail. Inside the broodsacs (sporocysts) are hundreds of microscopic larvae (see the picture on the top left), which infect the finicky bird. The parasite doesn’t harm the bird too much; otherwise the bird would be more careful when hunting for caterpillars.
morning, the ant rushes out of its nest, goes a meter from the ant-pile, and climbs up a blade of grass. At the top, it chomps into the grass and holds on. There it waits until noon, when it climbs down and finds But when I returned to the University in 1991, I had no inkling that I might study something like this. As I mentioned, my primary goal was to form a molecular phylogenetics lab. Above all I tried to get back into normal academic life in the University, but for some reason I could not attain it. I’m not sure, to what extent it was tied to my wandering between the University and the Institute of Academy of Sciences, or to the first symptoms of a mid-life crisis, or to the unexpected arrival of freedom for former Czechoslovakia and the feeling that whatever the future had in store for me, it could not be a wonderful as the fall of Communism. Probably a little bit of each. In 1987, after finishing my graduate study, I had to leave the University, which couldn’t find a place for me. Other graduates, who, unlike me, were members of the Communist party, were easily accommodated. But I wouldn’t say that reason I “left” was primarily political. Rather, the people in power tried to get rid of independent thinkers, who might someday pose a threat, or at least stand in contrast with the authority’s mediocrity. I have no illusions that this happens only in countries with Real Socialism. Nevertheless, there and then, leaders had an automatic system for chasing out such people – independent thinkers usually weren’t members of the Communist party, so it was easy to get rid of them under this pretext. In my case, it was probably also a matter of common human envy. At the time, I was invited to an excellent laboratory in a California university, where my more publication-successful competitors were studying the Trichomonas virus. And I, still young and naîve, mentioned in front my department head, who “coincidently” was also the leader of the local Communist party cell, what kind of pay I’d be getting at this American laboratory. As a result, I had to find a position outside the University. With a measure of good luck, I finally got a job in the Institute of Molecular Genetics at the Czechoslovak Academy of Sciences, in the laboratory of Vladimír Holáň. But my departure from Charles University lost me the opportunity to go the USA and continue researching Trichomonas viruses. In retrospect, I think it might have been for the better, because otherwise I wouldn’t have begun studying the manipulation hypothesis, nor made the acquaintance of Toxoplasma (however, I say this primarily for the benefit of that department head; if she happens to read this, I hope she stews in her envy). At my new workplace, I was officially working in biotechnology, developing the already-developed monoclonal antibodies for pregnancy tests (in other words, this is what I got paid for). Unofficially, I did genetic engineering
I wasn’t too happy in the academic institute, probably because I had been spoiled by the atmosphere in the University. To say it more positively, in my time at the College of natural sciences, I was most grateful for the fact that people on different research teams helped each other, borrowed each other materials and equipment, gave advice and most of all, had a true interest in their work – an interest which often even bordered on enthusiasm. They did science for the sake of science, not for money or their career. In the Institute, I got to study something I was interested in (by that I mean the semi-illegal part of my work), and I had incomparably better equipment than at the University, but I felt out of place. There was great rivalry among the research teams, and when I needed to borrow a certain chemical from a friend, I had to creep into his lab after working hours, so that his boss wouldn’t see. And so it’s not surprising, that immediately after the fall of Communism, I happily returned to my former position at the University. But to my dismay, soon after I got the unshakable feeling that I no longer belonged there. You can never step into the same river twice. Fortunately, it all straightened out after a couple years, but the first few years back in the College were strange and certainly not pleasant. Maybe for this reason, for the first time in my life, I began paying attention to my own psyche. I began considering past events in which my reactions had surprised me, but which I’d had neither the time nor the inclination to dwell on. And certain recent behavior patterns did not fit my personality. A simple example: buying salami sold by weight. In my childhood, butcher and delicatessen shops – and actually, pretty much any shop – that sold goods by weight, cheated their customers. Today, of course, they still do, but impersonally and on a professional basis – the cashier scans your vacuum-sealed bag of ham with added water, wishes you a good meal and hopes you’ll come again (or you can go to another store, if you think their water is better). During my childhood, salespeople apparently considered it a matter of personal pride, to cheat their customer of a couple ounces on each portion they sold. So when a person bought ten ounces of ham, he could count on either receiving less of the meat, or at least getting the wrong amount of change – or possibly, both. Of course, this also happened to me. And instead of protesting and causing a nice ruckus (and losing the possibility of returning to the store), I kept quiet. And what’s more, keeping quiet for some reason seemed more beneficial. As if staying mum and accepting the scam would somehow gain me a future advantage over the salesman. “I sure fooled him, letting him cheat me for the salami.” All in all, completely irrational behavior, which in retrospect, try as I might, I cannot see as beneficial. And after leaving the store, I could not understand the motive behind my actions, nor the feelings that accompanied them. Could the salesperson have hypnotized me? Now for a different example. The house I live in had bad electrical wiring, so time to time I blew a fuse. One day I was particularly successful, and blew not only the secondary circuit breaker, but also the main circuit breaker, and finally the power inlet box on the outside of my house. I knew that there are high currents running through this area, and that it’s safer not to approach it, but rather to call the professionals. I also happened to know that replacing this fuse positioned in front of the electricity meter is the business of Electrical Company (or whatever its name is) and that it’s free of charge. And I told myself – few people know that it‘s free of charge, so when the repairmen arrive, they’ll probably ask to be paid. No doubt they’ll say that I caused the damage, therefore I should pay for their trip and the broken fuse. And I was right. The contractors arrived, changed the fuse, and told me to pay them ten bucks. At the time, five bucks was something like fifty bucks today. I knew that they had no right to the money; and what’s more, I had anticipated their maneuver. But precisely because I knew it was a scam, and they thought that I didn’t know, then, with a feeling of superiority, I gave them those ten dollars. All the while, I told myself: there, that’ll show them. Of course, I didn’t show them anything – there was nothing to show. The contractors cheerfully stuck the ten dollars into their pocket and drove off. And I stood there in disbelief, waiting for the punch line. How is it possible, that whenever I let somebody cheat me, I have a strong feeling that I’m actually winning? At that moment, I understood that that feeling of superiority is only illusion, and incompatible with reality. The person who cheats me leaves with a feeling of smug satisfaction (he’d probably be even more pleased to know that I let him cheat me) and I am left only with the strange feeling, that I cannot grasp how I let it happen, and why on earth I thought it was a good idea. Like I said, after my return to the College of natural sciences, I started to see this pattern of strange behavior as an interesting psychological problem. I asked around, whether others had experienced something similar, and discussed the problem with my friends. I inquired whether anyone ever got the feeling, that in a particular situation they behave according to a foreign will and foreign interests, even though it seems like they are acting voluntarily. We probably discussed hypnosis and suggestion, since I thought they might be related. We tossed around the idea that certain people could be able to impose their will upon other people, so that victim behaves (with a feeling of superiority) in a way that benefits the suggester. I don’t recall what we concluded, but it was clear that none of my acquaintances had experienced this situation. About a month after one such debate, played out in a cozy, poorly lit corner of a pub close to the college building, Jaroslav Kulda stopped in my lab, in dire need of volunteers to test a new Toxoplasma antigen. For a long time, the department of parasitology conducted a study of toxoplasmosis. When I arrived in the College of natural sciences, this study was nearing its end, but a small part of original activities still existed, namely the testing Toxoplasma antigens. The method for producing this antigen for toxoplasmosis diagnosis was probably developed by the former researchers in the department of parasitology. By the time I arrived, the antigens had long been commercially produced by the Institute of Sera and Vaccines; but whenever they came out with a new batch of antigens, it brought them to our department for testing. In addition, several clinics sent us pregnant women suspected of having toxoplasmosis, and we were to screen them using a method known as the skin test. In this test, a Toxoplasma antigen (a mixture of macromolecules obtained by solubilization of Toxoplasma cells) is injected into the skin. Within two days, people infected with the parasite – who are Toxo positive – get a red spot surrounding the injection site. Based on the size of the spot (scientifically-speaking, based on the intensity of the delayed hypersensitivity reaction), we could determine how long ago a person had been infected, as well as the risk of health complications for a pregnant woman (see Box 57 How dangerous is Toxoplasma in pregnant women?). Testing antigens and screening pregnant women went well together; we had a regular supply of fresh antigen to test, and simultaneously a regular stream of patients who we could screen using the antigen. But before screening the women, we had to quickly determine how the antigen worked, and in what concentration we’d use it. And that was when Jaroslav Kulda ran from lab to lab, looking for volunteers for antigen testing. Today, of course, it would probably be impossible for a parasitologist who was not a physician to inject people with an antigen, but back then, they weren’t so strict about it. The times were a bit wilder, and such things weren’t as tied down by various regulations. For example, in one of the labs for the parasitology class, students injected each other with antigens to test for toxoplasmosis. In 1993, we even had an undergrad student in her second or third year of study conducting the skin test. I get goose bumps when I imagine the mess we’d be in if someone had complained about it, or even reported us to the authorities. For this reason, we quickly abandoned this method of testing, and switched over to the classic serological method. Serological tests, which determine the level of antibodies in a blood sample, are neither as sensitive nor as specific as a skin test, which means that one risks a false positive or false negative result (see Box 7 The specificity and sensitivity of diagnostic tests).
On the other hand, the researcher doesn’t risk get in getting trouble. One of our doctor friends, who came to the college once or twice a year, would draw (and still does) the blood for testing. The student volunteers would enter the “office” in four-minute – in later years, even three-minute – intervals, sign the informed consent form, get about 4 mL of blood drawn by the doctor, and pick up a psychological questionnaire to fill in. And we were lucky to have the doctor we did, because just his name guaranteed that the students would come in droves to have their blood drawn. Whenever I lured the students to this testing, I always emphasized that the doctor’s name was Zdeněk Hodný – something like “Joe Gentle” – and therefore that the blood draw wouldn’t hurt a bit. The students probably didn’t believe me, so they came to see for themselves. To get back to the start of our research: when Jaroslav Kulda came looking for volunteers, I naturally stepped forth. In the first place, I was interested in the testing process; and furthermore, in whether or not I was infected. To my surprise – and by that, I don’t mean pleasant – I found, two or three days after the testing, that I am Toxo positive. A red spot had formed around the injection site on my arm, and based on its size, I’d been infected relatively recently. At the time, I suspected this had happened in Japan, where I’d sampled raw or undercooked meat, and where the Toxoplasma prevalence is about the same as in the Czech Republic – that is, around 30%. But today I think my infection probably happened before my Japan trip, maybe when I was handling hay from a rabbit run (see Toxoplasma risk factors, discussed in chapter XXI). At the time of my testing, I knew little about Toxoplasma and toxoplasmosis (the disease caused by this parasite). Although I worked in the department of parasitology, and even in the room where Toxoplasma research was conducted; and my undergraduate work was carried out on protozoans in a lab shared by the department of parasitology and the department of animal physiology, my research focused on a different protozoan, the sexually-transmitted parasite Trichomonas. In any case, by education I was more of a physiologist or cellular physiologist; at heart an evolutionary biologist; and in terms of professional experience, primarily a molecular and cellular biologist. And as I mentioned, I’d spent four years working in
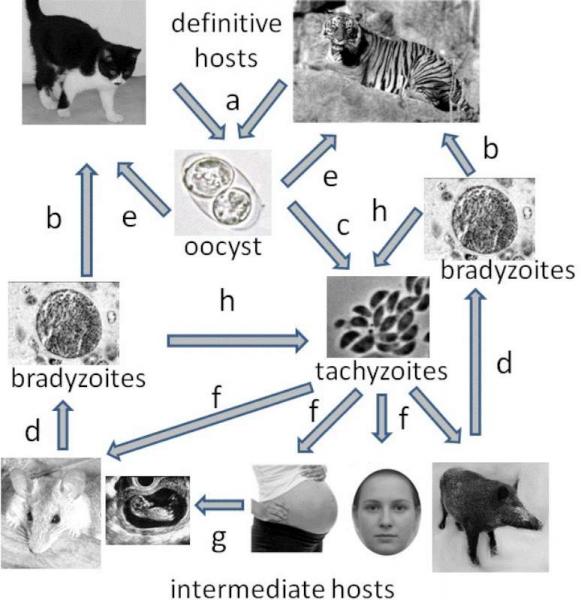
Fig. 6 The life cycle of Toxoplasma gondii. The definitive felid host releases oocysts in his feces (a). Oocysts reside in the soil and enter intermediate (c) and definitive hosts (e). In their bodies the oocysts quickly become tachyzoites (d), which divide rapidly. Tachyzoites spread the infection through the entire body (f), often using the host’s free-moving cells, such as white blood cells, as vectors. In host tissues, particularly in muscular, nervous, and connective tissue, tachyzoites change into slowly dividing bradyzoites (d), which permanently remain in the host in the form of tissue cysts. If the infected individual is eaten (b), then the tissue cysts release bradyzoites, which change into tachyzoites in the new host (h). If the consumer is felid, then the parasite differentiates in its intestinal cells. The tachyzoites first become merozoites and finally gametocytes, which fuse in pairs and form resistant oocysts. In a pregnant woman (or gravid female of any host species), tachyzoites can infect the developing fetus (g). Humans can be infected with oocysts from things like unwashed vegetable; or with bradyzoites from sources like raw or improperly cooked meat. Other sources of infection include contaminated water, and blood transfusion or organ transplantation from infected donors. immunological labs. The most I knew about Toxoplasma was that it’s some protozoan; but I sometimes mixed it up with Toxocara, which is a parasitic helminth (or, as an old-timer would say, a parasitic worm). When I discovered that I was Toxoplasma-infected, I quickly got to know my enemy. The first thing that caught my attention was the interesting life cycle of this parasite (Fig. 6). Toxoplasma is a coccidium, and thus related to the protozoan which causes malaria. Both Toxoplasma and malaria belong to the group known as the Apicomplexa. Toxoplasma reproduces sexually in the intestinal epithelium of felids. Cats, therefore, are Toxoplasma’s definitive hosts (see Box 8 Intermediate and definitive hosts).
Toxoplasma’s intermediate host, on the other hand, can be any warm-blooded animal – so under normal circumstances, a mammal or bird. It’s rumored that under “abnormal” circumstances, a fish can also become a Toxoplasma host, if kept at warm enough temperatures. It probably wouldn’t work with a carp – I doubt he’d appreciate water at 30 or more degree Celsius (86°F) – but with a tropic fish, it might be feasible. Toxoplasma’s life-cycle usually begins when an infected cat, the definitive host, excretes cysts in its scat. A cat is usually infected as a kitten, and releases cysts for only two or three weeks, although in large amounts. Then it stops releasing cysts, and thereafter is not contagious. Through the feces, the cysts make their way into the soil. From there they can infect any organism, be it mouse or man, that digs in the dirt, or eats something with dirt still on it. (For example, a child playing in the sandbox, or an adult munching on a carelessly-washed carrot). When a mouse becomes infected, it usually suffers only a mild illness (although this depends on how “bad,” or virulent, the strain of Toxoplasma is, and how many parasites got into the mouse); something similar happens with a human. Certain mammals get sicker than others. Upon reaching the intestines of an intermediate host (mouse or man), the protozoan exits the cysts and form tachyzoites, a motile form of Toxoplasma. Tachyzoites attack various cells in the body, and inside, they quickly reproduce. A healthy immune system deals with these tachyzoites pretty quickly. It develops a strong immune response, and the tachyzoites have to “retreat into illegality.” The quickly-reproducing tachyzoites become slowly-reproducing bradyzoites, which remain in the body as tissue cysts until the host’s death. If the infected intermediate host is eaten by a felid, the protozoan begins sexually reproducing in the cells on the internal surface of the intestines, and releasing hardy cysts into the feces. And so it comes full circle. If the intermediate host (mouse or man) is eaten by an organism other than a felid, it behaves just as it would in any intermediate host, reproducing only asexually and eventually forming tissue cysts. Immediately after infection, a person undergoes a phase known as acute toxoplasmosis, during which the tachyzoites are rapidly reproducing. In someone with a healthy immune system, this
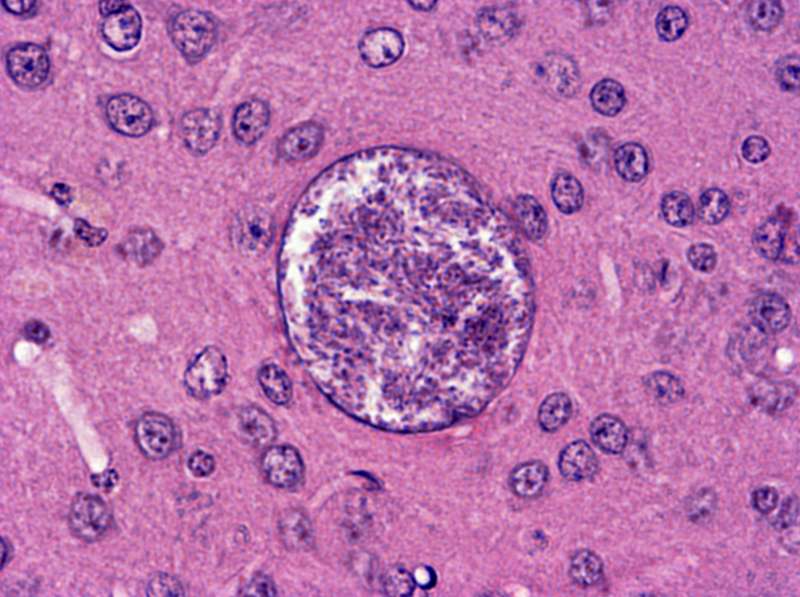
Fig. 7 A Toxoplasma cyst in a murine brain. The small particles inside the cyst are individual bradyzoites, “silently” awaiting the moment when the host is eaten by a predator (hopefully by a felid, the definitive host of Toxoplasma). The bradyzoites will be released by the action of digestive enzymes and penetrate the host from the intestinal lumen through the endothelium. Photo by Mirka Pečálková-Berenreiterová resembles mild virosis, and is often accompanied by swelling of the lymph nodes. The following phase, in which the parasites withdraw in tissue cysts, is known as latent toxoplasmosis. From a clinical standpoint, it is symptomless. Only in a small fraction of cases does a person ever realize that he was infected, and that he will carry Toxoplasma tissue cysts in his body for the rest of his life (Fig. 7). When reading up on Toxoplasma, one thing in particular caught my notice. This parasite can serve as a model organism for studying the manipulation hypothesis, because it seems that Toxoplasma alters the behavior of its intermediate host in order to up its chances of getting into a definitive host. In the 80s, an English team led by notable parasitologist William M. Hutchison (who discovered the life-cycle of Toxoplasma) tested this hypothesis. They published a number of studies showing that, in certain situations, Toxoplasma-infected mice behave differently than healthy mice. And in a natural setting, some of the behavioral differences of uninfected mice could have made them more likely to get eaten by the definitive host, a cat. It wasn’t
long before this new knowledge about Toxoplasma’s “life-style” clicked with what I’d had observed regarding my own behavior. In my muscle and nervous tissues, lies a protozoan whose interests are undoubtedly different from my own. Whereas evolution programmed me to survive and reproduce, it programmed Toxoplasma to try to manipulate me into getting eaten by a felid. Well, at least in the case of mice it can do so by altering their behavior (see Box 9 Manipulative parasites: body snatchers). So why couldn’t it also manipulate a human? Hidden in its tissue cysts, Toxoplasma has no way of knowing that it’s in a human instead of a mouse brain; and even less so, of knowing that, for several millennia now, the chances of humans becoming cat chow are very slim. (I wanted to write that the chances are non-existent; but not long after I started my studies, one of our students was killed by a tiger in the Prague Zoo. I don’t know whether the student was Toxo positive, seeing as he climbed into the enclosure, I wouldn’t be surprised.) So Toxoplasma continues doing what it learned from its evolution, from those tens of millions of years that it’s lived on this planet, attacking warm-blooded animals. It lies in its cysts, awaiting its opportunity, and manipulating its intermediate host so that he’ll quickly wind up in a felid’s stomach. Suddenly I had an explanation for my strange behavior, for those situations in which I sought to be the victim of an attack and voluntarily cooperated with the person doing me harm. Even this irrational behavior is, from an evolutionary standpoint, an advantageous adaptation – of course, it’s not good for me, the unwilling host of Toxoplasma, but it’s very useful for the parasite trying to get into a felid’s body. All the pieces fit. And I realized that, if my hypothesis were correct, then its implications applied not only to myself, but to an enormous amount of people. A third of the world’s population was Toxo positive in the early 90s. In the Czech Republic, it was also a third; in France, 60 to 70%; in Germany and Hungary, 50 to 60%; and in USA, about 20%. Although in China, or at least in those areas covered by the studies, the prevalence of toxoplasmosis is significantly lower – usually between 5 and 10% – in South America more than 60% of the population is infected, and in parts of Africa, over 90%. Just the number of potentially afflicted people is very disturbing; without taking into consideration the possible health and economic impacts. At this point, my primary concern wasn’t just my own behavior, but rather the possible effects of Toxoplasma on a third of the world’s population. And I immediately began planning, how to attack my hypothesis.
|